vapor liquid equilibria|vapor liquid equilibrium for distillation : Baguio For each component in a binary mixture, one could make a vapor–liquid equilibrium diagram. Such a diagram would graph liquid mole fraction . See more
Resultado da Teste seus conhecimentos sobre The Owl House! 🦉 ⚠️ Este quiz contém spoilers da primeira temporada de The Owl .
0 · vapor liquid equilibrium sample problems
1 · vapor liquid equilibrium phase diagram
2 · vapor liquid equilibrium pdf
3 · vapor liquid equilibrium for distillation
4 · vapor liquid equilibrium definition
5 · vapor liquid equilibrium database
6 · vapor liquid equilibrium data collection
7 · vapor liquid equilibrium calculator
8 · More
web22 de fev. de 2005 · Summary House (aka House MD), from executive producers Paul Attanasio, Katie Jacobs, David Shore, and Bryan Singer is a new take on mystery, where .
vapor liquid equilibria*******In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which . See moreThe field of thermodynamics describes when vapor–liquid equilibrium is possible, and its properties. Much of the analysis depends on whether the vapor and liquid consist of a single component, or if they are mixtures. See more
Binary mixture VLE data at a certain overall pressure, such as 1 atm, showing mole fraction vapor and liquid concentrations when . See morevapor liquid equilibria vapor liquid equilibrium for distillationAt boiling and higher temperatures the sum of the individual component partial pressures becomes equal to the overall pressure, which can . See more• Distillation Principals by Ming T. Tham, University of Newcastle upon Tyne (scroll down to Relative Volatility)• Introduction to Distillation: Vapor Liquid Equilibria See more
For each component in a binary mixture, one could make a vapor–liquid equilibrium diagram. Such a diagram would graph liquid mole fraction . See more• Continuous distillation• Dortmund Data Bank (includes a collection of VLE data)• See more In the case of a liquid enclosed in a chamber, the molecules continuously evaporate and condense, but the amounts of liquid and vapor do not change with time. . Vapor pressure is the result of the dynamic equilibrium that exists in every liquid. Read on to learn about what goes on at a microscopic level when you look a . This equation gives the relationship between the saturation vapor pressure and specific (molar) thermodynamic properties of the .Two types of vapor–liquid equilibrium diagrams are widely used to represent data for two-component (binary) systems. The first is a “temperature versus x and y” diagram (Txy). .
Fugacity. We use fugacity to represent equilibrium because fugacity is directly related to Gibbs free energy G (see eqn 11.30 in textbook). In an equilibrium between two phases .
The basis of distillation is phase equilibrium—specifically, vapor–liquid equilibrium (VLE) and in some cases vapor–liquid–liquid equilibrium (VLLE). Distillation can only
This Demonstration shows phase equilibrium for a binary system of two partially miscible liquids, A and B. Because of the partial miscibility, vapor-liquid equilibrium (VLE), liquid-liquid equilibrium .The vapor-liquid equilibrium is essential to present solvent properties that affect the performance of a CO2 capture process. The VLE reflects the absorption heat, cyclic . Calculates material flow and draws flowsheet of chemical batch processes using process simulation. Draw vapor-liquid equilibrium (VLE) and liquid-miscibility (LLE) phase diagrams, or calculate .With. P = Pressure at equilibrium P m S = Saturation pressure of the mixture P i S = Saturation pressure of component i (note : it is a function of the temperature) x i = molar fraction of the component in the LIQUID .Microsoft Word - Review VLE.doc. Vapor Liquid Equilibrium (VLE): A Guide. 10.213. Spring 2002. 04/29/02. YT. Here is a somewhat more systematic approach to VLE. There will not be much derivation from first principle. I suggest that you go through the derivation done in lecture notes or the textbook, once you are comfortable with the material here.Let us consider liquid-vapor equilibrium in a system with complete liquid miscibility, using as example the Zn-Mg system. Curves of gv and gl can be drawn at any given T and P, as in the upper panel of Fig. 5.3, and the common tangent construction then gives the equilibrium vapor and liquid compositions. The phase diagram depends upon the .
10.11: Vapor-Liquid Equilibrium. When a liquid such as water or alcohol is exposed to air in an open container, the liquid evaporates. This happens because the distribution of speeds (and hence kinetic energies) among molecules in a liquid is similar to that illustrated for gases, shown again below.Description. Vapor-Liquid Equilibrium, Second Edition covers the theoretical principles and methods of calculation of equilibrium conditions from various experimental data and the elements of measuring technique, as well as the instruments for the direct determination of the equilibrium compositions of the liquid and vapor phases of the system. Vapor-liquid equilibrium (VLE) defines the distribution of chemical species between the vapor and liquid phase and is an important criterion for process engineering applications. The thermodynamics of phase equilibrium is essentially similar to chemical equilibrium that is based on the minimization of Gibbs energy at a given pressure and .
Vapor-liquid equilibrium (VLE) data of the three binary systems of [C n mim]Br + H 2 O (n = 2, 3, 4) have been measured. The NRTL and e-NRTL models can be well used for calculation of the investigated systems and the absolute average relative deviations (AARD %) are 2.69% and 2.97%.Two types of vapor–liquid equilibrium diagrams are widely used to represent data for two-component (binary) systems. The first is a “temperature versus x and y” diagram (Txy). The x term represents the liquid composition, usually expressed in terms of mole fraction. The y term represents the vapor composition. The second diagram is a plot of

12.6.1 Miscibility in binary liquid systems. When two different pure liquids are unable to mix in all proportions, they are said to be partially miscible. When these liquids are placed in contact with one another and allowed to come to thermal, mechanical, and transfer equilibrium, the result is two coexisting liquid mixtures of different . Vapor-liquid equilibrium is a critical factor in common processes in the chemical industry, such as distillation. Distillation is the process of separating liquids by their boiling point. A liquid mixture is fed into a distillation unit or column, then boiled. Vapor-liquid equilibrium data is useful for determining how liquid mixtures will .
Figure 8.7.1 8.7. 1: The liquid phase is represented at the top of the graph where the pressure is higher. Oftentimes, it is desirable to depict the phase diagram at a single pressure so that temperature and composition are the variables included in the graphical representation. In such a diagram, the vapor, which exists at higher . The phase equilibrium between vapor and liquid is of significant importance in separation by distillation. Thorough knowledge of vapour-liquid equilibrium and multicomponent mixtures is necessary to understand, develop, and improve industrial distillation processes. Most of the correlations are empirical or semi-empirical and for . Dear Colleagues, This Special Issue covers topics on the vapor–liquid equilibrium (VLE) from both a theoretical and experimental viewpoint. Molecular simulations (e.g., density functional theory and molecular dynamics) and experimental techniques that focus on the equilibrium compositions and the effect of temperature .Isobaric Vapor–Liquid Equilibrium for Binary Systems of Allyl Alcohol with Water, Methanol, and Ethanol at 101.3 kPa. Journal of Chemical & Engineering Data 2016 , 61 (6) , 2071-2077.
Figure 8.7.1 8.7. 1: The liquid phase is represented at the top of the graph where the pressure is higher. Oftentimes, it is desirable to depict the phase diagram at a single pressure so that temperature and composition are the variables included in the graphical representation. In such a diagram, the vapor, which exists at higher . The phase equilibrium between vapor and liquid is of significant importance in separation by distillation. Thorough knowledge of vapour-liquid equilibrium and multicomponent mixtures is necessary to understand, develop, and improve industrial distillation processes. Most of the correlations are empirical or semi-empirical and for .
Dear Colleagues, This Special Issue covers topics on the vapor–liquid equilibrium (VLE) from both a theoretical and experimental viewpoint. Molecular simulations (e.g., density functional theory and .Isobaric Vapor–Liquid Equilibrium for Binary Systems of Allyl Alcohol with Water, Methanol, and Ethanol at 101.3 kPa. Journal of Chemical & Engineering Data 2016 , 61 (6) , 2071-2077. When a solution is heated, the liquid will evaporate or boil to form vapor. If the liquids are immiscible, then the phase diagram will show equilibrium betwe.
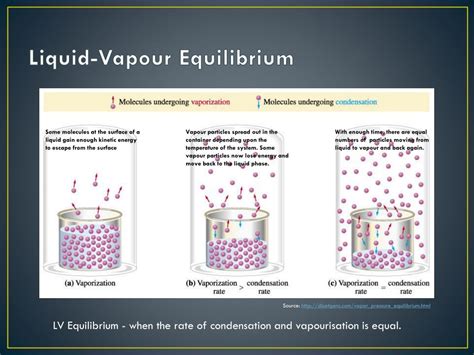
Vapor Liquid Equilibrium. Vapor-liquid equilibrium (known as VLE) is the state in which the rate of condensation is equal to the rate of evaporation so there is no net vapor-liquid interconversion . Typically, a substance in vapor-liquid equilibrium is at its boiling point. A boiling point diagram for a binary mixture is found to the right. .3.1 Introduction. Multiphase and solution thermodynamics deal with the composition of two or more phases in equilibrium. Thus, the maximum concentration of a species in an aqueous stream in contact with an organic stream can be estimated by these calculations. This can establish the contaminant levels obtained in various wastewater streams.Vapor-liquid equilibrium, abbreviated as VLE by some, is a condition where a liquid and its vapor (gas phase) are in equilibrium with each other, a condition or state where the rate of evaporation (liquid changing to vapor) equals the rate of condensation (vapor changing to liquid) on a molecular level such that there is no net (overall) vapor-liquid .Here, we consider vapor/liquid equilibrium of mixtures; see Figure 7.3 (page 180). Let x. i - mole fraction of component i in the liquid phase y. i - mole fraction of component i in the vapor phase The simplest case is an ideal liquid mixture and ideal gas where Raoult’s law states that for any component i, the partial pressure p. i = y. iVapor-Liquid Equilibria Using UNIFAC: A Group-Contribution Method focuses on the UNIFAC group-contribution method used in predicting quantitative information on the phase equilibria during separation by estimating activity coefficients. Drawing on tested vapor-liquid equilibrium data on which UNIFAC is based, it demonstrates through examples .The vapour-liquid Equilibrium (VLE) is a state where a substance’s liquid and vapour phases are in Equilibrium. The VLE is determined by the following factors: temperature, pressure, and the composition of the liquid and vapour phases. If any of these factors are changed, it will shift the Equilibrium, and the design of the steps will change .vapor liquid equilibria The basis of distillation is phase equilibrium-specifically, vapor-liquid equilibrium (VLE) and in some cases vapor-liquid-liquid equilibrium (VLLE). This chapter briefly reviews the fundamentals of VLE. One of the most important issues involved in distillation calculations is the selection of an appropriate physical property method that .
Resultado da Rules. You must be 18 or older to use this site. Do not post anything illegal under US law. Do not threaten or advocate violence. Do not sexualize minors.
vapor liquid equilibria|vapor liquid equilibrium for distillation